In a rapidly growing market, the EvidentIQ offering stands out as it combines a comprehensive tech stack with proven scientific services to support pharmaceutical companies, CROs and sponsor teams throughout the feasibility and clinical outcome data collection phase of their clinical trials.
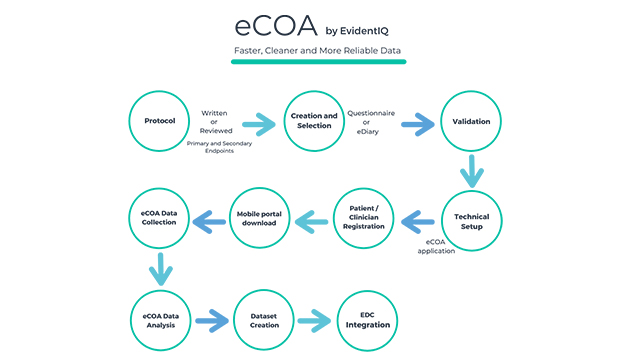
With its comprehensive eCOA solution, the new data science group, which has only recently been built by merging long-established providers XClinical, Carenity and Fortress Medical, has set out to fuse data science services and a scalable software platform. EvidentIQ customers benefit from an end-to-end eCOA package allowing to collect any patient / clinician / observer reported outcome data in a streamlined, compliant and efficient way. The solution can be used stand-alone or fully integrated into existing eClinical technologies, such as EDC systems. The EvidentIQ eCOA offering supports a bring-your-own-device (BYOD) policy and works on any iOS/Android phone.
EvidentIQ’s combined offering of a platform with a scientific service offering attached to it empowers LifeScience professionals to conduct virtual and hybrid clinical trials. Unique eFeasibility solutions help optimize clinical trials and accelerate patient recruitment with a patient-centric approach.
EvidentIQ relies on Data Science and robust technologies to custom the design of clinical trials optimizing Product Profiles, Endpoints, Protocols, ICFs, and eCRFs. The package facilitates a complete digitalized patient enrollment journey for clinical trials from Multi-channel Pre-screening over eConsent Management to the Clinical Data Capture in the EDC.